What Parasitic Way of Life Can Be Best Demonstrated by the Feeding Adaptations of What
Abstract
In host-parasite coevolutionary arms races, parasites probably have an evolutionary advantage. Parasite populations should be locally adapted, having higher mean fitness on sympatric than allopatric hosts. Here we assess evidence for local parasite advantage. Further we investigate how adaptation and counter-adaptation of parasites and hosts, necessarily occurring in sympatry, can generate a pattern of local adaptation. Already simple frequency-dependent selection models generate complex patterns of parasite performance on sympatric and allopatric populations. In metapopulations, with extinction, recolonization, and gene flow, variable selection pressure and stochasticity may obscure local processes or change the level at which local adaptation occurs. Alternatively, gene flow may introduce adaptive variation, so differential migration rates can modify the asymmetry of host and parasite evolutionary rates. We conclude that local adaptation is an average phenomenon. Its detection requires adequate replication at the appropriate level, that at which the local processes occur.
Conventional wisdom: parasites are locally adapted
It is conventional wisdom that parasites with relatively short generation times evolve faster than their hosts and are therefore ahead in the coevolutionary race, quickly overcoming new host resistance strategies. This process should lead to local adaptation, where a parasite population has higher mean performance on local vs. foreign host populations (Lively, 1996; Gandon & Van Zandt, 1998; Mopper & Strauss, 1998). Local adaptation may also be defined as the noninvasibility of a parasite population by competing foreign parasites, but throughout this paper we consider the former definition. Parasite performance or fitness does not necessarily covary with degree of damage to the host, though a widely used fitness estimate is infection success (Appendix). Here we use 'local/sympatric' and 'foreign/allopatric' to indicate the geographical scale at which parasite adaptation is detectable. Parasites may adapt to 'local' hosts at the scale of the individual, population or region, depending on the properties and dynamics of a given system. Allopatric hosts are those with which parasites are not coevolving.
Local adaptation of parasites was first demonstrated for herbivorous insects performing better on natal than on foreign host trees (Edmunds & Alstad, 1978), and adaptive deme formation in plant-herbivore systems has become a well-supported phenomenon (Van Zandt & Mopper, unpubl.; Mopper & Strauss, 1998). In fact, higher performance on local hosts occurs in several other host-parasite systems (Appendix), and has thus been considered a general rule (Ebert & Hamilton, 1996). Clearly, adaptive deme formation in plant–herbivore systems may not always involve an explicit arms-race scenario since trees are evolutionarily static compared to short-lived insects, but it can nonetheless be viewed as an end of an continuum with comparable evolutionary rates of host and parasite (or even the reverse) at the other end.
Although a common result, local adaptation of parasites is not universal. Half of the studies listed in the appendix did not detect parasite local adaptation, or even found the reverse pattern. We are currently conducting a meta-analysis to determine the generality of local adaptation of parasites. Clearly, when evolutionary rates do not differ because generation times or recombination rates are similar, parasites have no evolutionary advantage over their local hosts. Here we discuss mechanisms producing, masking, or even reversing the pattern of locally adapted parasites. In particular, we focus on the question of how the process of adapting to local hosts translates into a geographical pattern of local adaptation.
Process vs. pattern of local adaptation
The distinction between process and pattern of adaptation is not trivial. The former compares mean fitness of parasite populations before and after selective response within their host populations, while the latter compares performance on local hosts with that on foreign hosts.
Although it is clear that parasites with higher evolutionary potential should be better at exploiting their local hosts faster than these hosts can respond, it is not obvious how this process transforms into a geographical pattern (higher mean fitness on sympatric than on allopatric hosts). Most treatments consider adaptation of a parasite to its host population as a specialization process, with the often implicit assumption of a trade-off reducing performance on foreign hosts (Clarke, 1979). Parasite performance on allopatric hosts, then, should increase with genetic similarity between local and foreign hosts (Ebert, 1994). However, without additional assumptions about genetic mechanisms underlying specialization, or the genetic composition of foreign host populations, a geographical pattern is not a priori evident. Since coevolution only occurs in sympatry, one might equally argue that parasite performance on allopatric host populations is entirely unpredictable. Local adaptive changes may allow increased performance in some foreign host populations, leave performance unchanged in others, and diminish performance in still others. Therefore, adaptation by the parasite to its local host may not produce a pattern of local adaptation depending on the allopatric hosts compared.
The standard argument
In host-parasite arms races, parasites can increase their mean fitness by specializing on the most common host genotype in their local population. However, adaptation of the parasite to the common host type creates rare resistant host type advantage (Haldane, 1949; Hamilton, 1980), thus allowing counter-adaptation by the host. Population-genetics models show that such frequency-dependent selection generates time-lagged cycles of hosts and parasites with particular resistance and infectivity, respectively (Clarke, 1979; Hutson & Law, 1981; Bell & Maynard Smith, 1987; Nee, 1989; Hamilton et al., 1990). What is the relationship between these frequency-dependent cycles within populations and the geographical pattern of local adaptation among populations?
Nee's (1989) one-locus-two-allele model of hosts and parasites shows that host and parasite allele cycles can be described by two sine curves, with a phase shift depending on their relative evolutionary potential (Fig. 1, see also Dybdahl & Lively, 1995). When host and parasite have equal evolutionary potential (i.e. equal generation times, mutation and recombination rates) hosts with the common (frequency >0.5) allele are over-infected half the time, but under-infected the other half of the time (Fig. 1a) because of the time-lag in the frequency of the matching parasite genotype. Parasite populations cycle between high mean fitness periods (over-infection of a common clone) to low mean fitness periods (common genotypes under-infected; Fig. 1c).
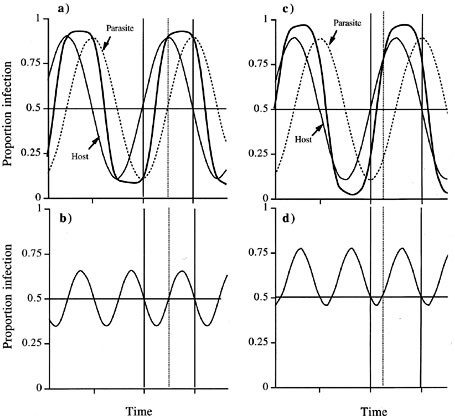
(a) Cycling frequency of a host allele and its corresponding parasite allele in a one-locus-two-alleles model (Nee, 1989; Dybdahl & Lively, 1995). Host and parasite alleles are 90° out of phase. The bold line presents the degree of over-and under-infection of the host allele at any time, i.e. whether this allele is proportionately over (under) infected in the population (calculated as the (fH1×fP1)/(fH1×fP1+fH2×fP2), fH and fP being frequencies of the host and parasite alleles, respectively. The solid vertical lines border the time during which the host allele is common (frequency >0.5), the dotted vertical line marks the transition from under-to over-infection of this host allele while it is common. (b) Cycling of mean parasite fitness calculated as the proportion of the two host alleles infected: (fH1×fP1+fH2×fP2). (c) as in (a), but the parasite evolves faster and is thus only 45° out of phase. (d) as in (b), with parasite only 45° out of phase.
By increasing the mutation/recombination rate or by decreasing generation time relative to the host parasites gain higher evolutionary potential, thereby decreasing the phase difference (Fig. 1b). Then appropriate parasite alleles increase in frequency more rapidly to track the spreading host allele. Consequently, hosts with the common host allele suffer over-proportional infection more of the time (Fig. 1b), and mean parasite fitness is high more often than low, although parasite fitness still oscillates (Fig. 1d).
Can these processes of adaptation within individual populations of parasite and host produce patterns of local adaptation? Consider several isolated populations with identical host and parasite alleles and dynamics (i.e. with identical sine functions). These populations, however, occupy different positions of their cycles at any time. When hosts and parasites have the same evolutionary potential, average infection rates between sympatric and allopatric combinations do not differ (Fig. 2a). However, when the parasite tracks the host more closely, sympatric infection rates exceed, on average, allopatric ones (Fig. 2b). That is, when parasites have an evolutionary advantage, an average pattern of local adaptation will be generated simply by isolated populations occupying different positions of their coevolutionary oscillations; even when different populations share the same alleles. When populations contain private alleles for resistance and virulence local adaptation should be more evident. Frequency-dependent cycling, then, should generally produce the pattern of local adaptation. Extending this model to several resistance and infectivity loci and Lotka–Volterra population dynamics, Morand et al. (1996) find a general sympatric parasite advantage over a broad range of intrinsic host growth rates and parasite transmission rates for similarly isolated populations.
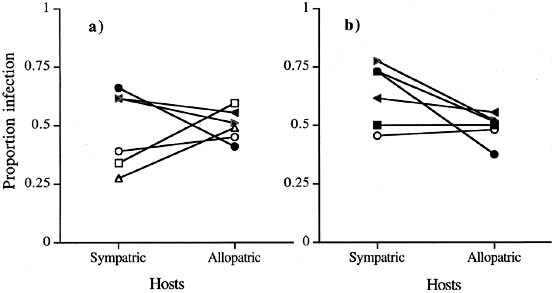
Proportion of infection of six parasite populations on sympatric and allopatric hosts for six populations; 'populations' consisted of the same four alleles with the same cycling dynamics, but at different (randomly determined) positions of their cycles. Populations were 'sampled' at a given time, and using the frequencies of each allele in each population parasite fitness (proportion infection, see 1b) was calculated for each possible combination of host and parasite population. Lines illustrate the difference in fitness on sympatric vs. allopatric hosts for each of the six parasite populations. Allopatric combinations represent averages across five allopatric host populations. Open symbols represent populations where the common host allele is under-infected. (a) Host and parasite alleles are 90° out of phase. (b) parasite only 45° out of phase.
Few studies have investigated patterns of over-and under-infection of common host genotypes. (Chaboudez & Burdon, 1995) found that in 13 out of 16 populations of the clonal plant Chondrilla juncea only locally common genotypes were infected with the rust fungus Puccinia chondrillina. In the topminnow (Poeciliopsis monacha), common gynogenetic triploid clones had higher infection rates than less common clones or sexual fish (Lively et al., 1990). In the freshwater snail Potamopyrgus antipodarum infected with trematodes (Dybdahl & Lively, 1995) and daphnia infected with several different microparasites (Little & Ebert, 1998), common clones were more infected than rare clones in some populations, but less or similarly infected in others, suggesting that these populations were in different positions in their cycles. Following one P. antipodarum population over time revealed that parasites drive host genotype oscillations, and parasites were better at infecting recently common than recently rare genotypes (Dybdahl & Lively, 1998). In the plant Arabis holboellii there was no correlation between host commonness and disease incidence with a pathogenic rust fungus in a field survey (Roy, 1993). However, in a transplant experiment rare or foreign genotypes of this plant experienced on average more rust and herbivore attack than common or local ones, indicating local host adaptation (Roy, 1998).
Equivocal results from natural populations are not unexpected. Even simple models predict variation in mean parasite performance on local hosts during coevolutionary host–parasite cycling (Fig. 1c,d). By chance, different populations will be in different phases of their cycles, so that patterns of local adaptation vary among populations (Fig. 2). Moreover, even for common locally adapted parasites, there may occasionally exist more suitable allopatric hosts. Therefore, for local adaptation in a host-parasite system to be detected populations are by necessity the unit of observation and replication will be required on two levels. First, performance of a given parasite population on its sympatric host population must be compared with that on several allopatric host populations to account for variation in suitability among different allopatric hosts. Second, replication of sympatric combinations of parasite and host is needed to account for the temporal variation of host-parasite dynamics within populations.
The genetic basis of resistance and virulence
Above, we presented a scenario of local adaptation arising from time-lagged cycles of host and parasite allele frequencies (Nee, 1989). How much do such cycles depend on the underlying genetic basis of resistance and virulence traits?
Nee's model employs a matching-allele system where any parasite type can only match (i.e. infect) a particular host type. This type of specificity produces the link between specialization on the local host and the pattern of local adaptation: tracking the sympatrically common host allele reduces a parasite's performance on foreign hosts if different clones are common in different populations. Cycling has also been obtained in multilocus matching-allele models with complex population dynamics, e.g. (Hutson & Law, 1981; Hamilton et al., 1990; Frank, 1996; Morand et al., 1996).
However, more realistically, a given parasite type may be capable of attacking several or all host types in a population. Gene-for-gene systems, typical of plant-pathogen interactions (Thompson & Burdon, 1992; Parker, 1996) allow fixation of universally virulent or resistant types, and therefore generate cycles only under conditions of high costs for the resistance and virulence alleles (Parker, 1994). Hence, in contrast with the pure frequency-dependent selection processes with matching alleles, additional genetic trade-offs are required to link local specialization and reduced performance on allopatric hosts.
Clearly, the coevolutionary arms race is difficult to envisage without some sort of frequency-dependent selection. But can local adaptation arise without ongoing time-lagged oscillations of the same alleles? Recent studies show that cycling is also possible for polygenic, quantitative traits that are constantly changing by mutation (Dieckmann et al., 1995; Gavrilets, 1997). Below we discuss the conditions for local adaptation in a metapopulation context where within-population frequency-dependent cycles are affected by local extinction and gene flow.
Local adaptation in a metapopulation context
Placing host-parasite interactions in a spatial or explicit metapopulation context is a relatively recent idea (e.g. Thompson & Burdon, 1992; Antonovics et al., 1994; Thompson, 1994; Thrall & Burdon, 1997). Host and parasite interactions take place in finite, subdivided populations that exchange genes in heterogeneous environments. This may lead to complex interactions between intra-and interpopulation processes over space and time (Thompson, 1994).
Metapopulations are groups of populations characterized by extinction and recolonization, and linked by gene flow. Most metapopulation models concentrate on the role that extinction and recolonization play in stabilizing coexistence and maintaining selected genetic variation in host and parasite (Frank, 1993; Ladle et al., 1993; Antonovics et al., 1994; Thrall & Jarosz, 1994a; Thrall & Jarosz, 1994b; Judson, 1995; Frank, 1996). Extinction/colonization processes of parasite and host in a metapopulation system depend on subpopulation size, the underlying genetic basis of the interaction between parasite and host, and the scale at which both players migrate between populations.
Clearly if host (and therefore parasite) populations experience high rates of local extinction, either because of small population size or because the suitable habitat itself is ephemeral, they may be recolonized by a genetically different pool than was previously present. Such systems, driven mainly by migration–drift dynamics, have limited possibility for coevolutionary interactions generating local adaptation. Similarly, if only parasite populations suffer high extinction rates, migration must be sufficiently high for recolonization to guarantee long-term coexistence. High migration itself may then further preclude local differentiation, and shifts in the genotype composition of the parasite will be caused by colonization–extinction dynamics entirely unrelated to local coevolutionary processes. Non-systemic parasites or those of hosts with nonoverlapping generations are likely to suffer regular local extinctions (Thrall & Burdon, 1997), and therefore may not show clear patterns of local adaptation.
Temporal and spatial variation has been extensively studied in populations of the perennial wild flax (Linum marginale) and the nonsystemic rust fungus Melampsora lini (Burdon & Jarosz, 1991; Jarosz & Burdon, 1991; Burdon & Jarosz, 1992; Burdon & Thompson, 1995). The metapopulation was dominated by few virulence types. Within population temporal fluctuations of pathogen virulence types were apparently unrelated to the resistance structure of the host populations (Burdon & Jarosz, 1991; Jarosz & Burdon, 1991). Similarly, changes in the composition of resistance types in host populations could not be attributed to changes in pathogen race composition (Burdon & Thompson, 1995). After a host population crash during an epidemic of the pathogen a marked decrease occurred in the abundance of relatively resistant host phenotypes, the opposite of what would be expected if the pathogen was the relevant selective force (Burdon & Thompson, 1995). Two explanations for such between-season fluctuations are possible. First, they may be truly stochastic and represent the consequence of local extinction and recolonization events. Alternatively, changes in the genetic composition of host or parasite could result from selection for other traits not involved in host-parasite coevolution. Non-adaptive change in disease resistance to the pathogen Synchytrium decipiens was also observed in a population of Amphicarpaea bracteata (Parker, 1991). In this predominantly self-pollinating plant, selection on traits linked with resistance genes was considered the most likely explanation (Parker, 1991).
Spatially explicit models
A simulation model of sets of populations, each with its local dynamics, linked by migration, and characterized by extinction and recolonization, found that the relationship between population disease levels and their mean resistance can be positive or negative, depending on the duration of the coevolutionary interaction (Thrall & Antonovics, 1995). In a young metapopulation, i.e. less than 50 generations old, there is a positive correlation between disease and resistance frequency, because disease selects for increasing resistance. In older metapopulations, this relationship becomes negative, because highly resistant host populations are unlikely to be colonized by disease. This negative correlation becomes established if there is a low cost of resistance, if the disease is rapidly lost as resistance spreads, if the population turnover is rapid, and if the disease is widespread.
What can be inferred from this result about the pattern of local adaptation? Regardless of whether populations come from old or young metapopulations, if populations are sampled at random one is likely to find a range of conditions. Some parasites, those from highly infected populations in long established metapopulations or from populations of low disease frequency in newly established metapopulations, will be better able to infect their local hosts that have less disease resistance than foreign hosts from population with different disease and resistance frequency. Therefore, when host parasite coevolution takes place in a metapopulation the overall pattern of local adaptation that may emerge from intrapopulation cycling of resistance and susceptible types with their, respectively, virulent parasites can be effaced by the metapopulation dynamics.
Fluctuations in the genetic composition of host and parasite populations may not preclude local adaptation. Populations may be long-lived enough to allow local coevolutionary processes. In this case, one may expect a complex interplay between stochastic processes, migration and selection. Especially in systems with many different resistance and virulence types (Frank, 1997) rare types may be lost by drift. Loss of a particular resistant host type will allow rapid spread of the corresponding parasite type, until such a host genotype is reintroduced by immigration. Consequently different populations may be in different states of local adaptation at any time. However, in contrast with the scenario of independent regular within-population cycles described above, these frequency-dependent selection processes should be strongly affected by (occasional) extinction/immigration events. Of course, if migration completely swamps local dynamics, local adaptation may only be apparent at higher geographical scales. Parasites migrating freely through a number of host populations with varying frequency of different resistance types then might adapt to the overall metapopulation frequency of particular host genotypes (Dybdahl & Lively, 1996). In this case one should test parasite performance on 'local' vs. 'foreign' host metapopulations.
A question of scale
Some host-parasite systems show a pattern of local adaptation over a scale of meters (Parker, 1985; Lively & Jokela, 1996), while in other systems this is found only for far larger scales. Although host resistance phenotype frequencies did not correspond to pathogen race frequencies in the Linum–Melampsora system (Burdon & Jarosz, 1991; Jarosz & Burdon, 1991), cross-inoculation tests for sympatric and allopatric combinations of pathogen and host suggest local adaptation (Burdon & Jarosz, 1991; Jarosz & Burdon, 1991; Burdon & Thompson, 1995), at least at the regional scale (Burdon & Thompson, 1995). Similarly a trypanosome parasite of bumble bees (Bombus terrestris) showed no within-region local adaptation, but tended to be maladapted at the regional level (Imhoof & Schmid-Hempel, 1998). Scale insects also revealed a survival advantage on their natal trees only when compared with performance on distant but not nearby host trees (Hanks & Denno, 1994).
Other conflicting interactions may be similar to hosts-parasite interactions. Cytoplasmic male sterility genes and their specific nuclear restorers in plants have evolutionary trajectories dominated by metapopulation dynamics (Frank, 1997). Local adaptation of nuclear restorer genes to a particular cytoplasmic male sterility type was present on scale of meters within a large continuous population of Plantago lanceolata (Van Damme, 1986). No such pattern of local adaptation was found over large geographical scales investigating populations separated by tens to hundreds of kilometres in Thymus vulgaris, another species with naturally occurring cytoplasmic male sterility (Gigord et al., 1998).
Migration and evolutionary potential
Several authors have stressed the importance of gene flow as a force introducing novel or lost resistance/virulence types into populations (Ladle et al., 1993; Thompson, 1994; Judson, 1995; Gandon et al., 1996; Frank, 1997). In fact, the relative rates of migration may be a decisive factor in the coevolutionary arms race. Even though parasites may have a general evolutionary advantage over their hosts, gene flow among host populations can introduce novel host genes that can counterbalance or even reverse this advantage (Thompson & Burdon, 1992; Thompson, 1994).
Gandon et al. (1996, 1998) have investigated the effects of differential migration of host and parasite on local adaptation. With a matching alleles system of resistance and virulence and stepping stone migration they found that, when parasites migrate more than their hosts, parasites can be locally adapted. On the other hand, when parasites migrate less than their hosts, they may be locally maladapted, i.e. less able to infect sympatric than allopatric hosts (Gandon et al., 1996). These patterns of adaptation or maladaptation are predicted to be highly variable over time because of the stochastic nature of the arrival of new favourable alleles. In large areas of parameter space defined by relative migration rates of hosts and parasites, no local pattern arises and parasites do not perform differently on sympatric vs. allopatric hosts (Gandon et al., 1998). This model also provides a theoretical formalization of the predicted decrease (or increase) of parasite performance with increasing geographical distance (and thus decreasing genetic similarity) between the host of origin and the allopatric host (e.g. Ebert & Hamilton, 1996).
Where parasite local adaptation and host parasite population structure have been measured we find qualitative concordance with this model. Trematode parasites migrate more than their snail hosts (Dybdahl & Lively, 1996) and are locally adapted (Lively, 1989). Conversely, the plant pathogen Microbotryum violaceum migrates less than its host plant Silene latifolia (Delmotte et al., submitted). In a cross-inoculation experiment this pathogen was less successful at infecting sympatric than allopatric hosts (Kaltz et al. unpubl./in prep.). However, restricted migration may not be the only factor contributing to local maladaptation of this pathogen. For example, the selfing breeding system in the parasite (vs. outcrossing of the host) may further limit the evolutionary potential of the parasites and cause population structuring (Delmotte et al. submitted). Lower migration rates of parasite than hosts may also explain local maladaptation of blood parasites of Canary Island lizards (A. Oppliger, pers. comm.) and reduced mortality effects of trypanosomes on bumble bees from the same region (Imhoof & Schmid-Hempel, 1998), although this largely vertically transmitted parasite may also benefit from low host damage.
Both negative and positive correlations between parasite performance and geographical distance of allopatric hosts (Ebert, 1994) would be a good indication that gene flow shapes patterns of local adaptation. However, empirical tests of this prediction are fraught with uncertainties. Too small a distance range may result in choice of genetically identical allopatric hosts. Furthermore geographical and genetic distances may not correlate highly (Davelos et al., 1996), and genetic distances estimated with neutral alleles may not reflect adaptive genetic differentiation (Carius and Ebert, unpubl.), rendering it impossible to order allopatric hosts correctly. Therefore, the combined use of neutral markers (to measure gene flow) and identification of virulence or resistance phenotypes (e.g. by testing them against a set of reference lines (Jarosz & Burdon, 1991)) may be advisable.
Environmental heterogeneity: yet another level of complication
So far, we have considered environmental effects only as stochastic forces potentially overriding selective processes. However, the coevolutionary dynamics themselves may be affected by environmental heterogeneity in more predictable ways, some of which generate serious pitfalls. Phenotypic variation has an environmental as well as a genetic component. Host and parasite performance may be influenced by epigenetic maternal or conditioning effects such as acquired immunity. Furthermore, parasites or hosts may perform better in their home site because they have adapted to their local environment rather than to their local hosts and parasites, respectively (Rice, 1983; Sork et al., 1993; Roy, 1998). Natural parasite populations may also have their own coevolving hyperparasites, so parasites may be locally adapted only in the absence of these hyperparasites (Mopper et al., 1995). Such additional environmental effects cause potential problems in interpretation of transplant experiments, so common garden experiments are often more appropriate (Karban, 1989).
Another complicating factor is that environments can differ in quality. High-quality habitats may have different evolutionary optima for defence strategies by hosts and offensive strategies by parasites (Hochberg & van Baalen, 1998). Evolution towards different optima under different environmental conditions may render interpretation of experimental studies difficult. In particular, performance of host and parasite in cross-inoculation experiments under common garden conditions may lead to equivocal conclusions about local adaptation. For example, with different costs of virulence and resistance in different environments, parasites from high-quality environments where high virulence is selected may appear locally maladapted, i.e. more successful on less defended allopatric hosts from poorer environments. But at the same time they appear locally adapted because of their superiority over less virulent parasites from low-quality environments. Additionally, high-quality environments may export migrants to poorer environments, and asymmetric gene flow along a natural productivity gradient (Lively & Jokela, 1996; Stanton & Galen, 1997) may swamp local adaptive processes.
What can we learn from this exercise?
- 1
Adaptation of parasites to their local hosts is a common phenomenon, but not universal, and sometimes the pattern is even reversed. A meta-analysis investigating the generality of local adaptation is currently under way.
- 2
If the main interest is to study coevolutionary dynamics, clearly the best approach is to observe populations over time to detect the process of adaptation. For certain systems (e.g. bacteria and viruses) this may be a valuable option even for laboratory experiments. However, following the dynamics in the field may be very time-consuming and technically challenging.
- 3
Therefore, to test whether the spatial differentiation of hosts and parasites observed in the field has resulted from processes of adaptation, we may have to study adaptation as a geographical pattern. Patterns will change over time and space, so the relevant unit of observation is the population (or deme), and local adaptation is likely to be detected only 'on average'. As with any experiment one can only determine the number of replicates needed to detect significant effects with a priori knowledge of the variation inherent in natural populations.
- 4
Metapopulation dynamics may strongly interfere with local selection processes. In certain systems strong drift-migration dynamics may prevent patterns of local adaptation, or shift the level of local adaptation to higher hierarchical levels, e.g. the regional level.
- 5
In the metapopulation context, migration may be an important factor influencing evolutionary rates of both host and parasite. A simple prediction is that higher rates of gene flow of one player may lead to local adaptation of that player (Gandon et al., 1996). Environmental heterogeneity may further complicate the process and pattern of local adaptation. Altogether, one may expect to find a geographical mosaic of varying degrees of local adaptation as a consequence of migration among populations from varying habitat quality (Thompson, 1994).
References
-
Ahmed, H. U., Mundt, C. C. and Coakley, S. M. (1995). Host-pathogen relationship of geographically diverse isolates of Septoria tritici and wheat cultivars. Plant Path, 44: 838–847.
-
Antonovics, J., Thrall, P. H., Jarosz, A. and Stratton, D. (1994). Ecological genetics of metapopulations: the Silene-Ustilago plant-pathogen system. In: L. E. Real (ed.) Ecological Genetics, pp. 146–170. Princeton University Press, Princeton, NJ.
-
Ballabeni, P. and Ward, P. I. (1993). Local adaptation of the trematode Diplostomum phoxini to the European minnow Phoxinus phoxinus its second intermediate host. Func Ecol, 7: 84–90.
-
Bell, G. and Maynard Smith, J. (1987). Short-term selection for recombination among mutually antagonistic species. Nature, 328: 66–68.
-
Bevan, J. R., Crute, I. R. and Clarke, D. (1993). Variation for virulence in Erysiphe fischeri from Senecio vulgaris. Plant Path, 42: 622–635.
-
Briskie, J. V., Sealy, S. G. and Hobson, K. A. (1992). Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution, 48: 334–340.
-
Burdon, J. J. and Jarosz, A. M. (1991). Host-pathogen interactions in natural populations of Linum marginale and Melampsora lini: II. Patterns of resistance and racial variation in a large host population. Evolution, 45: 205–217.
-
Burdon, J. J. and Jarosz, A. M. (1992). Temporal variation in the racial structure of flax rust (Melampsora lini) population growing on natural stands of wild flax (Linum marginale): local vs. metapopulation dynamics. Plant Path, 41: 165–179.
-
Burdon, J. J. and Thompson, J. N. (1995). Changed patterns of resistance in a population of Linum marginale and the rust pathogen Melampsora lini. J Ecol, 83: 199–206.
-
Carlsson-Granér, U. (1997). Anther-smut disease in Silene dioica: Variation in susceptibility among genotypes and populations, and patterns of disease within populations. Evolution, 51: 1416–1426.
-
Chaboudez, P. and Burdon, J. J. (1995). Frequency-dependent selection in a wild plant-pathogen system. Oecologia, 102: 490–493.
-
Clarke, B. C. (1979). The evolution of genetic diversity. Proc R Soc B, 205: 453–474.
-
Davelos, A. L., Alexander, H. M. and Slade, N. A. (1996). Ecological genetic interactions between a clonal host plant (Spartina pectinata) and associated rust fungi (Puccinia seymouriana and Puccinia sparganioides). Oecologia, 105: 205–213.
-
Dieckmann, U., Marrow, P. and Law, R. (1995). Evolutionary cycling in predator-prey interactions: Population dynamics and the Red Queen. J Theor Biol, 178: 91–102.
-
Dufva, R. (1996). Sympatric and allopatric combinations of hen fleas and great tits: a test of the local adaptation hypothesis. J Evol Biol, 9: 505–510.
-
Dybdahl, M. F. and Lively, C. M. (1995). Host-parasite interactions: infection of common clones in natural populations of a freshwater snail. Proc R Soc B, 260: 99–103.
-
Dybdahl, M. F. and Lively, C. M. (1996). The geography of coevolution: Comparative population structures for a snail and its trematode parasite. Evolution, 50: 2264–2275.
-
Dybdahl, M. F. and Lively, C. M. (1998). Host-parasite interactions: Evidence for rare advantage and time-lagged selection in a natural population. Evolution, in press
-
Ebert, D. (1994). Virulence and local adaptation of a horizontally transmitted parasite. Science, 265: 1084–1086.
-
Ebert, D. and Hamilton, W. D. (1996). Sex against virulence: the coevolution of parasitic diseases. Trends Ecol Evol, 11: 79–82.
-
Edmunds, G. F. and Alstad, D. N. (1978). Coevolution in insect herbivores and conifers. Science, 199: 941–945.
-
Ennos, R. A. and McConnel, K. C. (1995). Using genetic markers to investigate natural selection in fungal populations. Can J Bot, 73 (S1): S302–S310.
-
Failloux, A.-B., Raymond, M., Ung, A., Glaziou, P., Martin, P. M. V. and Pasteur, N. (1995). Variation in the vector competence of Aedes polynesiensis for Wuchereria bancrofti. Parasitology, 111: 19–29.
-
Frank, S. A. (1993). Coevolutionary genetics of plants and pathogens. Evol Ecol, 7: 45–75.
-
Frank, S. A. (1996). Statistical properties of polymorphism in host-parasite genetics. Evol Ecol, 10: 307–317.
-
Frank, S. A. (1997). Spatial processes in host-parasite genetics. In: I. Hanski and M. E. Gilpin (eds) Metapopulation Dynamics: Ecology, genetics, evolution, pp. 325–352. Academic Press, New York.
-
Gandon, S. and Van Zandt, P. A. (1998). Local adaptation and host-parasite interactions. Trends Ecol Evol, 13: 214–216.
-
Gandon, S., Capowiez, Y., Dubois, Y., Michalakis, Y. and Olivieri, I. (1996). Local adaptation and gene-for-gene coevolution in a metapopulation model. Proc R Soc B, 263: 1003–1009.
-
Gandon, S., Ebert, D., Olivieri, I. and Michalakis, Y. (1988). Differential adaptation in spatially heterogeneous environments and host-parasite coevolution. In: S. Mopper and S. Y. Strauss (eds) Genetic Structure and Local Adaptation in Natural Insect Populations, pp. 325–342. Chapman & Hall, New York.
-
Gavrilets, S. (1997). Coevolutionary chase in exploiter-victim systems with polygenic characters. J Theor Biol, 186: 527–534.
-
Gigord, L., Lavigne, C., Shykoff, J. A. and Atlan, A. (1998). No evidence for local adaptation between cytoplasmic male sterility and nuclear restorer genes in the gynodioecious species Thymus vulgaris L. Heredity, in press
-
Haldane, J. B. S. (1949). Disease and evolution. Ricerca Scientifica, 19: 68–76.
-
Hamilton, W. D. (1980). Sex vs. non-sex vs. parasite. Oikos, 35: 282–290.
-
Hamilton, W. D., Axelrod, R. and Tanese, R. (1990). Sexual reproduction as an adaptation to resist parasites. Proc Natl Acad Sci USA, 87: 3566–3573.
-
Hanks, L. M. and Denno, R. F. (1994). Local adaptation in the armoured scale insect Pseudaulacaspis pentagona (Homoptera: Diaspididae). Ecology, 75: 2301–2310.
-
Hochberg, M. E. and Van Baalen, M. (1998). Antagonistic coevolution over productivity gradients. Am Nat in press
-
Hutson, V. and Law, R. (1981). Evolution of recombination in populations experiencing frequency-dependent selection with time delay. Proc R Soc B, 213: 345–359.
-
Imhoof, B. and Schmid-Hempel, P. (1998). Patterns of local adaptation of a protozoan parasite to its bumblebee host. Oikos, 82: 59–66.
-
Jarosz, A. M. and Burdon, J. J. (1991). Host-pathogen interactions in natural populations of Linum marginale and Melampsora lini: II. Local and regional variation in patterns of resistance and racial structure. Evolution, 47: 1618–1627.
-
Judson, O. (1995). Preserving genes: a model of the maintenance of genetic variation in a metapopulation under frequency-dependent selection. Genet Res Cam, 65: 175–191.
-
Karban, R. (1989). Fine-scale adaptation of herbivorous thrips to individual host plants. Nature, 340: 60–61.
-
Kimberling, D. N. and Price, P. W. (1996). Variability in grape phyloxera preference and performance on canyon grape (Vitis arizonica). Oecologia, 107: 553–559.
-
Ladle, R. J., Johnstone, R. A. and Judson, O. P. (1993). Coevolutionary dynamics of sex in a metapopulation: Escaping the Red Queen. Proc R Soc B, 253: 155–160.
-
Little, T. J. and Ebert, D. (1998). Associations between parasitism and host genotype in natural populations of Daphnia (Crustacea: Cladocera). J Anim Ecol in press
-
Lively, C. M. (1989). Adaptation by a parasitic trematode to local populations of its snail host. Evolution, 43: 1663–1671.
-
Lively, C. M. (1996). Host-parasite coevolution and sex. Bioscience, 46: 107–114.
-
Lively, C. M. and Jokela, J. (1996). Clinal variation for local adaptation in a host-parasite interaction. Proc R Soc B, 263: 891–897.
-
Lively, C. M., Craddock, C. and Vrijenhoek, R. C. (1990). Red Queen hypothesis supported by parasitism in sexual and clonal fish. Nature, 344: 864–866.
-
Memmott, J., Day, R. K. and Godfray, H. C. J. (1995). Intraspecific variation in host plant quality: The aphid Cinara cupressi on the Mexican cypress Cupressus lusitanica. Ecol Ent, 20: 153–158.
-
Mopper, S. and Strauss, S. Y. (1998). Genetic Structure and Local Adaptation in Natural Insect Populations: Effects of Ecology, Life History, and Behavior. Chapman & Hall, New York.
-
Mopper, S., Beck, M., Simberloff, D. and Stiling, P. (1995). Local adaptation and agents of selection in a mobile insect. Evolution, 49: 810–815.
-
Morand, S., Manning, S. D. and Woolhouse, M. E. J. (1996). Parasite-host coevolution and geographic patterns of parasite infectivity and host susceptibility. Proc R Soc B, 263: 119–128.
-
Nee, S. (1989). Antagonistic coevolution and the evolution of genotypic randomization. J Theor Biol, 140: 499–518.
-
Parker, M. A. (1985). Local population differentiation for compatibility in an annual legume and its host-specific fungal pathogen. Evolution, 39: 713–723.
-
Parker, M. A. (1989). Disease impact and local genetic diversity in the clonal plant Podophyllum peltatum. Evolution, 43: 540–547.
-
Parker, M. A. (1991). Non-adaptive evolution of disease resistance in an annual legume. Evolution, 45: 1209–1217.
-
Parker, M. A. (1994). Pathogens and sex in plants. Evol Ecol, 8: 560–584.
-
Parker, M. A. (1996). The nature of plant-parasite specificity. Evol Ecol, 10: 319–322.
-
Rice, W. R. (1983). Parent-offspring pathogen transmission: A selective agent promoting sexual reproduction. Am Nat, 121: 187–203.
-
Roy, B. A. (1993). Patterns of rust infection as a function of host genetic diversity and host density in natural populations of the apomictic crucifer Arabis holboelli. Evolution, 47: 111–124.
-
Roy, B. A. (1998). Differentiating the effects of origin and frequency in reciprocal transplant experiments used to test negative frequency-dependent selection hypothesis. Oecologia, 115: 73–83.
-
Soler, M. and Møller, A. P. (1990). Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature, 343: 748–750.
-
Sork, V. L., Stowe, K. A. and Hochwender, C. (1993). Evidence for local adaptation in closely adjacent subpopulations of northern red oak (Quercus rubra L.) expressed as resistance to leaf herbivores. Am Nat, 142: 928–936.
-
Stanton, M. L. and Galen, C. (1997). Life on the edge: Adaptation vs. environmentally mediated gene flow in the snow buttercup Ranunculus adoneus. Am Nat, 150: 143–178.
-
Strauss, S. Y. (1997). Lack of evidence for local adaptation to individual plant clones or site by a mobile specialist herbivore. Oecologia, 110: 77–85.
-
Thompson, J. N. (1994). The Coevolutionary Process. University of Chicago Press, Chicago.
-
Thompson, J. N. and Burdon, J. J. (1992). Gene-for-gene coevolution between plants and parasites. Nature, 360: 121–125.
-
Thrall, P. H. and Antonovics, J. (1995). Theoretical and empirical studies of metapopulations: population and genetic dynamics of the Silene-Ustilago system. Can J Bot, 73 (S1): S1249–S1258.
-
Thrall, P. H. and Burdon, J. J. (1997). Host-pathogen dynamics in a metapopulation context: the ecological and evolutionary consequences of being spatial. J Ecol, 85: 743–753.
-
Thrall, P. H. and Jarosz, A. M. (1994a). Host-pathogen dynamics in experimental populations of Silene alba and Ustilago violacea I. Ecological and genetic determinants of disease spread. J Ecol, 82: 549–559.
-
Thrall, P. H. and Jarosz, A. M. (1994b). Host-pathogen dynamics in experimental populations of Silene alba and Ustilago violacea II. Experimental tests of theoretical models. J Ecol, 82: 561–570.
-
Vandamme, J. M. M. (1986). Gynodioecy in Plantago lanceolata L. V. Frequencies and spatial distribution of nuclear and cytoplasmic genes. Heredity, 56: 355–364.
Acknowledgements
We thank Minus van Baalen, Achim Carius, Mark Dybdahl, Sylvain Gandon, Jukka Jokela, Tom Little, Anne Oppliger, Bitty Roy, and Peter Van Zandt for providing data or unpublished manuscripts. Discussions with Minus van Baalen and Sylvain Gandon and with many people during the local adaptation workshop in Paris 1998, helped clarify our ideas. Comments by Bitty Roy, Dieter Ebert, Mark Dybdahl, Isabelle Olivieri and one anonymous reviewer improved a previous version of the manuscript. This work was supported by Swiss National Science Foundation Grant 31–33638.92.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Table 1
Rights and permissions
About this article
Cite this article
Kaltz, O., Shykoff, J. Local adaptation in host–parasite systems. Heredity 81, 361–370 (1998). https://doi.org/10.1046/j.1365-2540.1998.00435.x
-
Received:
-
Accepted:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1046/j.1365-2540.1998.00435.x
Keywords
- coevolution
- frequency-dependent selection
- metapopulation
- sympatry
- time-lagged cycles
Further reading
Source: https://www.nature.com/articles/6884350
0 Response to "What Parasitic Way of Life Can Be Best Demonstrated by the Feeding Adaptations of What"
Post a Comment